Details of the Drug
General Information of Drug (ID: DMLPAOB)
| Drug Name |
Allopurinol
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Adenock; Ailural; Ailurial; Allohexal; Allohexan; Alloprin; Allopur; Allopurin; Allopurinolum; Allorin; Allozym; Allpargin; Allural; Aloprim; Alopurinol; Aloral; Alositol; Aluline; Anoprolin; Anzief; Apulonga; Apurin; Apurol; Atisuril; Bleminol; Bloxanth; Caplenal; Capurate; Cellidrin; Cosuric; Dabrosin; Dabroson; Embarin; Epidropal; Epuric; Foligan; Geapur; Gichtex; Gotax; Hamarin; Hexanuret; Jenapurinol; Ketanrift; Ledopur; Lopurin; Lysuron; Milurit; Milurite; Miniplanor; Monarch; Nektrohan; Novopurol; Progout; Pureduct; Purinol; Remid; Riball; Rimapurinol; Roucol; Sigapurol; Suspendol; Takanarumin; Tipuric; Urbol; Uribenz; Uricemil; Uridocid; Uriprim; Uripurinol; Uritas; Urobenyl; Urolit; Urosin; Urtias; Xanthomax; Xanturat; Xanturic; Zygout; Zyloprim; Zyloric; Dura Al; Pan Quimica; A 8003; BW 56158; Urtias 100; AL-100; Allo-Puren; Allohexal (TN); Allopurinol(I); Allosig (TN); Apo-Allopurinol; BW 56-158; Ketobun-A; Progout (TN); Puricos (TN); Quimica, Pan; Zyloprim (TN); Zyloric (TN); BW-56-158; B. W. 56-158; 4-HPP
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antimetabolites
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
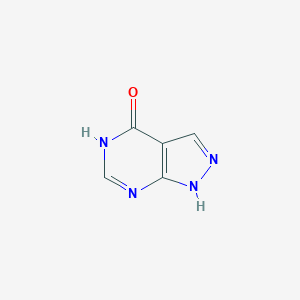 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 136.11 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Allopurinol
Coadministration of a Drug Treating the Disease Different from Allopurinol (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Allopurinol FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6795). | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Reiter S, Simmonds HA, Webster DR, Watson AR: On the metabolism of allopurinol. Formation of allopurinol-1-riboside in purine nucleoside phosphorylase deficiency. Biochem Pharmacol. 1983 Jul 15;32(14):2167-74. | ||||
| 5 | Schoenhard G, Oppermann J, Kohn FE: Metabolism and pharmacokinetic studies of misoprostol. Dig Dis Sci. 1985 Nov;30(11 Suppl):126S-128S. doi: 10.1007/bf01309397. | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 8 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 9 | Clinical Pharmacogenetics Implementation Consortium guidelines for human leukocyte antigen-B genotype and allopurinol dosing. Clin Pharmacol Ther. 2013 Feb;93(2):153-8. doi: 10.1038/clpt.2012.209. Epub 2012 Oct 17. | ||||
| 10 | A study of HLA class I and class II 4-digit allele level in Stevens-Johnson syndrome and toxic epidermal necrolysis. Int J Immunogenet. 2011 Aug;38(4):303-9. doi: 10.1111/j.1744-313X.2011.01011.x. Epub 2011 May 4. | ||||
| 11 | Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genomics. 2011 May;21(5):303-7. doi: 10.1097/FPC.0b013e32834282b8. | ||||
| 12 | Allopurinol: xanthine oxidase inhibitor. Tex Med. 1966 Jan;62(1):100-1. | ||||
| 13 | Isolation, characterization and differential gene expression of multispecific organic anion transporter 2 in mice. Mol Pharmacol. 2002 Jul;62(1):7-14. | ||||
| 14 | Renal transport of organic compounds mediated by mouse organic anion transporter 3 (mOat3): further substrate specificity of mOat3. Drug Metab Dispos. 2004 May;32(5):479-83. | ||||
| 15 | Xanthine oxidase inhibition by allopurinol affects the reliability of urinary caffeine metabolic ratios as markers for N-acetyltransferase 2 and CYP1A2 activities. Eur J Clin Pharmacol. 1999 Jan;54(11):869-76. | ||||
| 16 | Drug-induced hepatic steatosis in absence of severe mitochondrial dysfunction in HepaRG cells: proof of multiple mechanism-based toxicity. Cell Biol Toxicol. 2021 Apr;37(2):151-175. doi: 10.1007/s10565-020-09537-1. Epub 2020 Jun 14. | ||||
| 17 | Allopurinol induces innate immune responses through mitogen-activated protein kinase signaling pathways in HL-60 cells. J Appl Toxicol. 2016 Sep;36(9):1120-8. doi: 10.1002/jat.3272. Epub 2015 Dec 7. | ||||
| 18 | Allopurinol Protects Against Cholestatic Liver Injury in Mice Not Through Depletion of Uric Acid. Toxicol Sci. 2021 May 27;181(2):295-305. doi: 10.1093/toxsci/kfab034. | ||||
| 19 | Systemic drugs inducing non-immediate cutaneous adverse reactions and contact sensitizers evoke similar responses in THP-1 cells. J Appl Toxicol. 2015 Apr;35(4):398-406. doi: 10.1002/jat.3033. Epub 2014 Aug 4. | ||||
| 20 | Elion GB, Yu TF, Gutman AB, Hitchings GH "Renal clearance of oxipurinol, the chief metabolite of allopurinol." Am J Med 45 (1968): 69-77. [PMID: 5658870] | ||||
| 21 | Shah KA, Levin J, Rosen N, Greenwald E, Zumoff B "Allopurinol hepatotoxicity potentiated by tamoxifen." N Y State J Med 82 (1982): 1745-6. [PMID: 6960280] | ||||
| 22 | Lowenthal DT "The treatment of hyperuricemia." Am Fam Physician 14 (1976): 98-100. [PMID: 937167] | ||||
| 23 | Boelaert JR, Dom GM, Huitema AD, Beijnen JH, Lange JM "The boosting of didanosine by allopurinol permits a halving of the didanosine dosage." AIDS 16 (2002): 2221-2223. [PMID: 12409745] | ||||
| 24 | Berns A, Rubenfeld S, Rymzo WT "Hazard of combining allopurinol and thiopruine." N Engl J Med 286 (1972): 730-1. [PMID: 5061067] | ||||
| 25 | Petitpierre B, Perrin L, Rudhardt M, et al "Behaviour of chlorpropamide in renal insufficiency and under the effect of associated drug therapy." Int J Clin Pharmacol 6 (1972): 120-4. [PMID: 4638970] | ||||
